IV Protective Cap Ref.JRA01
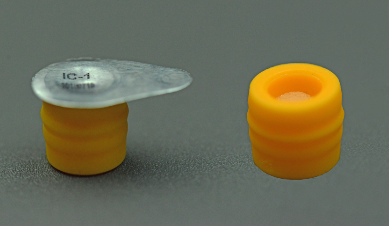
IV Protective Cap Ref.JRA01
Configuration: Aluminum foil, Cap-body, Foam ball, Isopropyl alcohol.
Functionality: Used for providing a physical barrier on a needleless injection site.
All components in manufacturing are used with conventional medical grade, biocompatible materials.
Sterilized by Gamma radiation before shipment.
A single-use device.
Previous:
Y Connector & Injection Site

